For the moment, three vaccines have been approved by the European Medicines Agency (EMA), those released by Pfizer-BioNTech, Moderna and AstraZeneca. The latter joined France's stable of approved Covid-19 vaccines most recently, with the first doses administered to healthcare professionals under the age of 65 on February 6. Each of the three vaccines has shown good results in terms of efficacy (more than 90 percent for the Pfizer and Moderna iterations), although France's Haute Autorité de Santé (HAS) health authority has cautioned against using the AstraZeneca vaccine in those over 65 due to a lack of sufficient data on its impact in older individuals. France will allow the AstraZeneca vaccine to be administered by pharmacists.
The difference between the vaccines lies primarily in how they need to be stored. The Pfizer-BioNTech vaccines must be kept at -80°C and used quickly after they come out of that deep freeze (within five days at most) while doses of the Moderna vaccine can be stored at -20°C and kept in a refrigerator for a month before injection. AstraZeneca doses can be kept in cold storage at temperatures between +2°C and +8°C.
What's the difference between the various Covid-19 vaccines available in France? How will getting inoculated work? And what happens after the jab? France's vaccination campaign has raised a wealth of questions. FRANCE 24 answers 20 of them here.
After a sluggish start, France’s Covid-19 vaccination campaign sharply accelerated in January, passing 1 million inoculations on January 23. Available first to nursing home residents and healthcare professionals over 50 years of age, the government extended vaccinations to people over 75 and anyone at high risk for Covid-19 starting January 18. The country approved a third vaccine for use in February and aims to vaccinate everyone who wants a jab by summer's end.
FRANCE 24 takes a closer look at France's vaccination effort as it rolls up its sleeves to put an end to Covid-19.

What vaccines are available in France and what are the differences between them?
Are the vaccines effective against the new Covid-19 variants?
The vaccines’ effectiveness against the new variants of Covid-19 remains to be seen. Researchers have said they are not concerned about effectiveness against the new strain first detected in the United Kingdom but the South African variant has raised more questions. Meanwhile, a strain detected in Brazil and Japan is "the most worrying of all", Ravi Gupta, a professor of clinical microbiology at the University of Cambridge, told AFP. Indeed, tests show that the variant could possibly escape from the antibodies meant to neutralise it, sidestepping the body's natural defence memory and the immunity that comes with it.
US biotechnology firm Moderna said on January 25 that lab studies showed its Covid-19 vaccine remained effective against variants of the coronavirus first identified in the UK and South Africa. That positive news was somewhat tempered by the finding a six-fold reduction in the level of highly potent neutralising antibodies produced against the South African variant (B.1.351). The company said it would try adding a second booster of its vaccine – meaning three shots in total – "out of an abundance of caution" and has begun preclinical studies on a booster specifically for the South African variant.
South Africa suspended the start of its inoculations with the AstraZeneca vaccine on February 8 after a trial showed it provided only "minimal" protection against mild to moderate Covid-19 caused by the variant first detected in that country. AstraZeneca told AFP it believed its vaccine would still protect against severe disease and said researchers were already working to update the vaccine to tackle the South African variant.
US biotechnology firm Moderna said on January 25 that lab studies showed its Covid-19 vaccine remained effective against variants of the coronavirus first identified in the UK and South Africa. That positive news was somewhat tempered by the finding a six-fold reduction in the level of highly potent neutralising antibodies produced against the South African variant (B.1.351). The company said it would try adding a second booster of its vaccine – meaning three shots in total – "out of an abundance of caution" and has begun preclinical studies on a booster specifically for the South African variant.
South Africa suspended the start of its inoculations with the AstraZeneca vaccine on February 8 after a trial showed it provided only "minimal" protection against mild to moderate Covid-19 caused by the variant first detected in that country. AstraZeneca told AFP it believed its vaccine would still protect against severe disease and said researchers were already working to update the vaccine to tackle the South African variant.
Why is the French vaccine slow in coming to market?
The results of the phase 1 and 2 trials unveiled in December of the vaccine developed by the French multinational Sanofi were positive for adults between the ages of 18 and 49, but the immune responses elicited in older subjects were deemed disappointing. According to Sanofi, a new trial with "an improved antigen formula" is set to begin in February. The third phase will not be able to begin before the second half of 2021, however, which pushes back the vaccine's eventual availability on the market to the end of this year, at best.
On January 25, France's Pasteur Institute, named for 19th-century French vaccine pioneer Louis Pasteur, announced it was ending development of a vaccine it was working on based on an existing measles vaccine with US pharmaceutical firm Merck after clinical trial results proved disappointing. The institute stressed it would be continuing its work on two other vaccine projects using different methods.
On January 25, France's Pasteur Institute, named for 19th-century French vaccine pioneer Louis Pasteur, announced it was ending development of a vaccine it was working on based on an existing measles vaccine with US pharmaceutical firm Merck after clinical trial results proved disappointing. The institute stressed it would be continuing its work on two other vaccine projects using different methods.

I've already had Covid-19. Do I need to get vaccinated?
There is currently no data on the potential benefits of being vaccinated against Covid-19 after having been infected, France's Haute Autorité de Santé (HAS) health authority has explained. Someone who has already contracted Covid-19 could still benefit from the vaccine, even if "the risk, even theoretical, among those people appears ... very low", France's Society for Infectious Pathology (SPILF) explained. In such cases, one must respect a minimum three-month wait after the onset of symptoms and not present persistent symptoms.
Should I be tested for Covid-19 before getting inoculated?
No, not unless there has been contact with someone who is sick. In the case of a previous Covid-19 infection, after consulting with one's doctor, waiting three months after the onset of symptoms is enough. But otherwise France's HAS does not recommend antibody testing to decide whether or not to be vaccinated because it "doesn’t provide proof of immunity to the virus".
Do I have a choice over what vaccine I will get?
Health Minister Olivier Véran has been fairly clear on the matter. "Today, we have two approved vaccines," he told BFMTV on January 7. "They are two messenger RNA vaccines that have the same efficacy, so there is no need to question the choice of vaccine." To be clear, it isn't possible in France to be picky about which jab one gets. "We aren't going to start with: 'For me, I prefer an attenuated vaccine and, me, I prefer an RNA vaccine...' We'd never manage," said Véran.
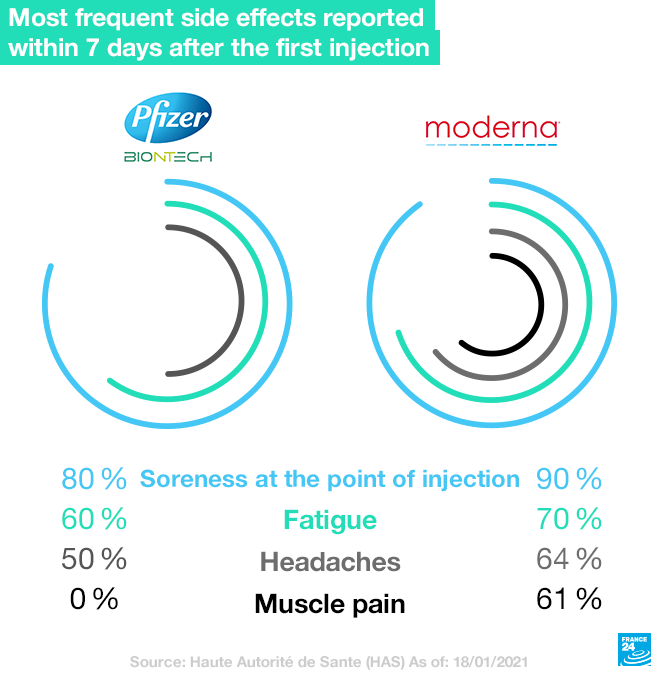
What are the side effects?
France's (HAS) has listed the most frequent episodes reported in the seven days following the first injection. With the Pfizer-BioNTech Cominraty vaccine, the most frequently observed side effects are soreness at the site of the injection (in 80 percent of cases), fatigue (in more than 60 percent of cases), headaches (in more than 50 percent of cases), as well as muscle pain and chills. However, these side effects were seen as mild and wore off in under 48 hours. With the Moderna vaccine, the main reactions observed also include soreness at the point of injection (more than 90 percent of cases), fatigue (70 percent), headaches (more than 64 percent) and muscle pain (more than 61 percent).
Have there been any serious side effects after the vaccines were administered?
During the Pfizer-BioNTech vaccine trials, out of the more than 43,000 people taking part in Phase 3 testing, four participants developed temporary facial paralysis, the causes of which are still being investigated, That was also the case for four participants in the Moderna vaccine trials (three in the vaccinated group, one in the placebo group) out of more than 30,000 people taking part in those tests. Overall, about 10 serious side effects were recorded during the trials. In France, since the start of the vaccination campaign, "six cases of serious side effects with a favourable trend were observed after the injection of the Comirnaty vaccine from Pfizer-BioNTech", France's National Agency for Medicine Safety (ANSM) said in a communiqué on January 14. The agency has committed to publishing the data collected by drug safety authorities on a weekly basis. The updates can be viewed in French here.
What medical follow-up is there after the injections?
As is the case with all medicines, doctors and pharmacists must report any negative side effect suspected of being related to the vaccine. Moreover, it is possible for a patient to signal potential concerns directly to their regional drug safety authority (CRPV) or report them online.
Multiple authorities, including Epi-Phare, are carrying out studies on those who receive vaccinations in order to identify possible side effects.
Multiple authorities, including Epi-Phare, are carrying out studies on those who receive vaccinations in order to identify possible side effects.
Can I still carry and transmit Covid-19 once I've been vaccinated?
There is no clear answer to this question as yet, although some data on the matter is promising. The Moderna vaccine, in particular, saw a lower number of asymptomatic patients (0.1 percent) in the vaccinated group compared to the control group (0.3 percent) after two injections. For AstraZeneca, a half-dose of the first injection protected recipients against asymptomatic infections by 58 percent.
However, further studies need to be carried out to answer the question with certainty. Indeed, France's health minister warned that the "vaccine protects from grave pulmonary forms, but that does not mean it protects from the nasal or oropharyngeal [throat] forms", as Véran told BFMTV.
However, further studies need to be carried out to answer the question with certainty. Indeed, France's health minister warned that the "vaccine protects from grave pulmonary forms, but that does not mean it protects from the nasal or oropharyngeal [throat] forms", as Véran told BFMTV.

Is the vaccine compatible with pregnancy and breastfeeding?
No adverse side effects were reported in pregnant women who took part in the clinical trials, be it with regard to the pregnancy itself or in the development of the foetus during the preclinical trials led by Pfizer and Moderna on animals. However, pregnant women are currently excluded from the vaccination campaign while targeted studies are conducted. According to the European Medicines Agency, vaccination will only be considered once the expected benefit exceeds the potential risk. As far as breastfeeding is concerned, there is not yet data on the vaccine’s potential to transfer into breastmilk after the mother is inoculated.

Why are the elderly first to be vaccinated?
The French government aims above all to ease the pressure on hospitals. Their strategy therefore is to prioritise those most vulnerable to Covid-19 and those most susceptible to developing serious forms of the illness. "This strategy leads to the biggest reduction in serious forms and deaths," the HAS said in late December. Older people have been the primary victims of Covid-19 since the start of the pandemic last year.
"We can vaccinate younger people but if turns out they are still transmitting the virus, there is no point," HAS chief Dominique Le Guludec told Franceinfo on January 12. "One must vaccinate those who are the targets, those they can give it to and those at risk of a severe form" of the illness, Le Guludec added.
"We can vaccinate younger people but if turns out they are still transmitting the virus, there is no point," HAS chief Dominique Le Guludec told Franceinfo on January 12. "One must vaccinate those who are the targets, those they can give it to and those at risk of a severe form" of the illness, Le Guludec added.
Who else is in the priority group?
Professionals working in nursing homes and presenting a high risk (those above the age of 50 or suffering from other conditions), healthcare professionals including those in private practice, firefighters, home care workers over the age of 50 or with existing medical conditions, are also among those considered priority candidates for Covid-19 vaccination in France. Indeed, "people resident in facilities are those most at risk for serious forms of the illness and these locations are known to be places where the virus circulates quickly," the health ministry has said. Professionals working in such facilities are therefore particularly exposed. As of January 18, people over 75 who live independently, as well as high-risk individuals under the age of 75, are also eligible for the jab.
What are the "high-risk" conditions that qualify someone for Covid-19 vaccination?
As French Prime Minister Jean Castex announced on January 14, more than 800,000 people "suffering from particularly serious illnesses" can now receive the vaccine. Those include people suffering from severe kidney failure, organ transplant recipients, cancer patients, and people with Down's Syndrome, Health Minister Véran specified. The official list also includes people suffering from certain rare illnesses.
How quickly does the French government want people vaccinated?
France’s vaccination campaign, which began on December 27, got off to a very slow start but sped up in January. It reached the million mark on January 23, according to health ministry figures. By February 6, more than 1.8 million people in France had received at least one dose of Covid-19 vaccine and nearly 250,000 were fully inoculated with both doses, accordingly to figures compiled by Santé Publique France (the public health agency) and Our World in Data.
On January 21, Health Minister Olivier Véran said France would be able to vaccinate 70 million people by the end of August "if all of the vaccines ordered are approved by European and global health authorities". He told TV channel TF1 that the number of people vaccinated in the country would progressively increase to "4 million at the end of February, 9 million in March, 20 million at the end of April, 30 million at the end of May, 43 million at the end of June, 57 million at the end of July, and 70 million, that is to say the entire French population, by the end of August".
However, Jean-François Delfraissy, who heads the scientific panel advising the French government on Covid-19, said he did not share the government's conviction that the population could be vaccinated in its entirety by the summer. "We will vaccinate a maximum number of people by mid-April, probably 6 to 8 million people", and have perhaps 40 percent of the population vaccinated by the end of the summer – "but no more", Delfraissy told BFMTV on January 24. He cited limitations on the pharmaceutical industry's capacity to produce vaccine doses in massive quantities.
On February 2, French President Emmanuel Macron promised that everyone in France who wants to be vaccinated against Covid-19 would be offered a shot by the end of the summer, which is to say September 22. In the interview broadcast on TF1, the French leader also said that the 80 percent of nursing home residents who wish to be vaccinated (some 500,000 people) will have their wish fulfilled by "the beginning of March". He said the country's vaccination campaign is "unfolding at the pace that was planned".
On January 21, Health Minister Olivier Véran said France would be able to vaccinate 70 million people by the end of August "if all of the vaccines ordered are approved by European and global health authorities". He told TV channel TF1 that the number of people vaccinated in the country would progressively increase to "4 million at the end of February, 9 million in March, 20 million at the end of April, 30 million at the end of May, 43 million at the end of June, 57 million at the end of July, and 70 million, that is to say the entire French population, by the end of August".
However, Jean-François Delfraissy, who heads the scientific panel advising the French government on Covid-19, said he did not share the government's conviction that the population could be vaccinated in its entirety by the summer. "We will vaccinate a maximum number of people by mid-April, probably 6 to 8 million people", and have perhaps 40 percent of the population vaccinated by the end of the summer – "but no more", Delfraissy told BFMTV on January 24. He cited limitations on the pharmaceutical industry's capacity to produce vaccine doses in massive quantities.
On February 2, French President Emmanuel Macron promised that everyone in France who wants to be vaccinated against Covid-19 would be offered a shot by the end of the summer, which is to say September 22. In the interview broadcast on TF1, the French leader also said that the 80 percent of nursing home residents who wish to be vaccinated (some 500,000 people) will have their wish fulfilled by "the beginning of March". He said the country's vaccination campaign is "unfolding at the pace that was planned".
Can children be vaccinated?
Not at the moment in France, due to the order of priority the HAS established in early December. In addition, manufacturers must first obtain authorisation for the lower age categories, which have not yet participated in clinical trials. Pfizer-BioNTech does not have authorisation for its vaccine to be administered to anyone under the age of 16, while recipients of the Moderna vaccine must be 18 or older. The former began a trial in October on participants aged between 12 and 15; the latter has yet to recruit candidates for its trial of 3,000 volunteers aged 12 to 17.

What about setting up a "vaccine passport"?
Even though the French government has repeatedly said the Covid-19 vaccine will not be mandatory, a potential "vaccine passport" could both break that promise and raise questions around personal freedom. Still, while the subject is controversial in France, HAS President Le Guludec suggested to Franceinfo that it isn't relevant at the moment. "We have not explored this issue because, quite simply, we don't know if this vaccine blocks transmission, so it doesn't make sense," she said.

Once I'm vaccinated, do I still have to wear a face mask?
Face masks remain mandatory even after vaccination and those inoculated must maintain social distancing. Studies are under way to explore the capacity of antibodies induced by the vaccine to rapidly project through nasal mucus before the virus can replicate there and be transmitted through the air to another person.
Will being vaccinated become mandatory for foreign travel?
France's transport minister said on January 11 that the answer to that was leaning towards "no". For the moment, in any case, no country is asking for a document proving one's Covid-19 vaccination status as a condition for entry. The World Health Organization (WHO) has said it is opposed "for the moment" to introducing Covid-19 vaccination certificates as a condition for entry to international travellers.

Will a new jab be necessary every year?
"It is possible that Covid-19 will become an illness with seasonal outbreaks like the flu, which would justify obtaining long-term immunity," France's Spilf has explained. Two factors will also be pertinent going forward: The duration of immunity that Covid-19 vaccines induce and the evolution of the virus. Indeed, if the body were no longer able to recognise certain strains of the virus, the vaccine would need to be updated, which would mean requiring subsequent vaccinations.